1087 Jamison Rd
Washington Court House, OH 43160
(800) 321-1562

Knowledge is key to using your analytic results to their fullest. The Spectrum Agronomic Library provides you with useful information that will help you to better understand the complex science of agronomy. Our agronomists will be continually adding original and reprinted articles, so check the library regularly for new information.
With the advent of grid sampling, many people are taking more soil samples, and paying more attention to them. Because of this, there are more questions about why soil labs get different results for the same field, and even the same sample, and why fertilizer recommendations can be significantly different between laboratories. There is a long, but reasonably simple explanation for these differences.
A soil sample report is really two distinct services:
Lab differences occur in both areas, so we must look at each area separately.
The following article was originally written by Greg Roth with Penn State University.
The variation in our weather from year to year and location to location has a substantial impact on corn silage quality in our region. This variation causes dairy producers and nutritionists the need to adjust rations to maintain high milk production levels. The accompanying table summarizes some of the effects of weather on yield and several forage quality traits of corn. Most of these data were adapted from a summary by Coors and Lauer (2001) from studies in the Netherlands and New York, but our data from Pennsylvania studies would support them as well.
| Table 1. Weather Effects on Corn Silage | ||||
|---|---|---|---|---|
| Factor | DM Yield | Digestibility | Fiber Content | Fiber Digestibility |
| High temperature | + | - | + | - |
| High light intensity | + | + | - | ± |
| High populations | + | - | + | ± |
| Delayed planting | - | - | + | ± |
| Delayed harvest | - | - | ± | - |
| Drought | - | - | + | + |
Negative (-), positive (+) and mixed (±) effects on the trait.
Higher temperatures can increase DM yield, but cause more respiration to occur late in the season and reduce grain fill. High light intensity (lack of cloudy days) promotes good grain fill that has positive effects on whole plant digestibility. As plant densities increase, yields often increase, but reduced sugars and starch in the silage may lead to slightly lower whole plant digestibility. Delaying harvest tends to reduce dry matter slightly, decrease digestibility, and here in Pennsylvania, often decreases the fiber content and fiber digestibility. Drought generally reduces yield, and grain content. This increases the fiber content but this is often accompanied by lower lignin production that increases the fiber digestibility. In seasons where the drought comes at the end of the season, sometimes the grain content is reduced but the stalks are large and well lignified, so the fiber digestibility is not increased.
Most people involved in crop production understand the importance of micronutrients in achieving higher and more profitable yields. However, simply applying a needed micronutrient to the soil does not mean that the crop yield will increase. The true benefit of any fertilizer nutrient is in the amount that is actually taken up by the crop. Yield gains come from the nutrients being in the correct chemical form, place, time, and amount that the crop requires. !lock
Crops take up only those nutrients that are dissolved in the soil solution. Therefore, the solubility of fertilizer sources equates to their availability and is critical to properly feeding a crop. An application of low solubility micronutrients does little good for the following crop. Farmers and fertilizer dealers can choose from many different sources for each micronutrient. These sources have different levels of water-solubility. Some, such as chelates also have additional characteristics that resist fixation or “tie-up” by the soil for extended periods of time.
Among the inorganic (non-chelate) sources in the market, the more common sources are sulfates, oxides, and oxysulfates. Sulfates such as zinc sulfate (ZnSO4) and manganese sulfate (MnSO4), are assumed to be 100% soluble. Because of the high solubility of sulfate forms, the associated micronutrients are highly available to the crop and are often considered the “benchmark” against which other sources are compared. Sulfate forms are produced by completely reacting the target nutrient with sulfuric acid, until there is only the sulfate form remaining.
| Table 1 : Corn Growth (% of maximum) | ||||
|---|---|---|---|---|
| Percent Water solubility | 5.0 lbs/acre | 10 lbs/acre | 20 lbs/acre | |
| Zinc Applied (lbs/acre) | ||||
| 5 | 61.7% | 62.4% | 72.5% | |
| 10 | 64.4% | 65.6% | 78.3% | |
| 15 | 66.8% | 68.5% | 82.9% | |
| 20 | 69.1% | 71.3% | 86.6% | |
| 25 | 71.3% | 73.9% | 89.5% | |
| 30 | 73.3% | 76.3% | 91.7% | |
| 35 | 75.2% | 78.6% | 93.5% | |
| 40 | 77.0% | 80.7% | 95.0% | |
| 45 | 78.6% | 82.6% | 96.1% | |
| 50 | 80.2% | 84.5% | 97.0% | |
| 55 | 81.7% | 86.2% | 97.7% | |
| 60 | 83.0% | 87.8% | 98.2% | |
| 65 | 84.3% | 89.2% | 98.7% | |
| 70 | 85.5% | 90.6% | 99.0% | |
| 75 | 86.6% | 91.9% | 99.3% | |
| 80 | 87.7% | 93.1% | 99.5% | |
| 85 | 88.7% | 94.2% | 99.7% | |
| 90 | 89.6% | 95.3% | 99.8% | |
| 95 | 90.5% | 96.2% | 99.9% | |
| 100 | 91.3% | 97.1% | 100.0% | |
Some micronutrient sources, such as zinc oxide (ZnO) are completely in the oxide form. Oxides are essentially insoluble in a single crop season, therefore unavailable to the following crop. There are some exceptions to this, such as when oxides are ground to an exceptionally fine powder and impregnated on fertilizer granules. Oxides are normally the lowest cost materials. However, because they are essentially insoluble, most agronomists consider them to be ineffective as a fertilizer source.
Oxysulfates are a combination of oxides and sulfates. They are produced similarly to sulfates, except that the acidulation is not complete. Oxysulfates contain variable proportions of oxide and sulfate forms of the micronutrient. The amount of each depends on how far the reaction is permitted to proceed toward completion. Their effectiveness falls between oxides and sulfates and can be highly variable. Oxysulfates that contain higher amounts of the sulfate form will be more effective. The price of oxysulfates normally falls between sulfates and oxides and is typically proportional to the amount of sulfate in the product. It is in this area of balancing the cost vs. effectiveness of oxysulfates that most confusion and problems can occur.
A 1999 study conducted by researchers at Colorado State University and Alberta Agriculture illustrates the relationship of solubility and effectiveness of different sources of micronutrients. They looked at the growth response of young corn to various sources and rates of Zn fertilizer grown in Zn deficient soils. The study included zinc sulfate (99.9% water soluble) and zinc oxysulfate sources with various solubility (0.7% to 98.3%). They found at a constant application rate, growth increased as the solubility of the Zn source increased. Also, in order to obtain equal growth from a low solubility source, up to 4 times as much fertilizer was needed, and sometimes even that was not enough. They concluded that in order to be effective, a Zn fertilizer must be at least 50% water-soluble.
The results of this work were consistent enough that the researchers were able to develop mathematical equations to reliably predict the Zn uptake from a given rate of Zn application, based on the solubility of the fertilizer. The results of these formulas are shown in Table 1.
When first evaluating the data in Table 1, we could conclude that applying 10 lb./ac of a 5% soluble material would get about 2/3 the result of applying the same rate of a 100% soluble material. Therefore, we should apply 33% more of the 5% soluble source to equal the 100% soluble source. However, the goal of fertilization is to get optimum growth response. Looking at the data in this way we see that in order to achieve more than 90% growth response, we have the roughly equivalent options shown in Table 2.
| Table 2 : Equivalent Effective Rates | |||
|---|---|---|---|
| Fertilizer Solubility | Application Rate (lbs/acre) | Effectiveness | Relative Value |
| 95% | 5.0 | 90.5% | 100% |
| 70% | 10.0 | 90.6% | 50% |
| 30% | 20.0 | 91.7% | 25% |
Table 2 indicates that we should apply twice the amount of a 70% soluble material, and 4 times the amount of a 30% soluble material, to achieve the same goal as the 5.0 lb./ac rate of the 95% soluble source. Based on this evaluation, the 95% soluble source is twice as effective as the 70% soluble source, and 4 times as effective as the 30% soluble source.
This relationship between solubility and effectiveness applies to other nutrients as well. In 1986, researchers at North Carolina State University, looking at manganese (Mn) sources used in row fertilizer, reported that “Manganese deficiency symptoms occurred with the least acid-forming starter fertilizer and decreased with increasing water solubility of the accompanying Mn materials. Application of Mn materials with water solubility <100% would require proportionally higher rates of application of Mn to maintain Mn in the leaf. The required Mn rate increases dramatically with decreasing water solubility of Mn material in the presence of a non-acid starter fertilizer.” Stated simply, the higher the solubility of the Mn source, the better it worked. It also confirmed that Mn is more available in acidic conditions created by some formulations of row fertilizer.
In “Micronutrients in Agriculture”, Soil Science Society of America, Dr. John Mortvedt states that the “Use of granular oxysulfates with bulk blends will require that close attention be given to the level of water-soluble micronutrients in these by-products to ensure that sufficient amounts of micronutrients are immediately available to plants.”
A complicating factor in using and selling micronutrients is that there are no Federal, and few State laws regulating the definition of materials that may be labeled as sulfates or oxysulfates. For example, a zinc fertilizer may be primarily in the sulfate form, but have a significant amount of the oxide form as well. Such a fertilizer may legally be labeled as zinc sulfate. These fertilizers would not be expected to have as much zinc in soil solution early in the season as a product that was 100% in the sulfate form. At this time, the only option available to dealers and growers questioning the solubility of a fertilizer is to have the fertilizer tested for water solubility.
Logic would dictate that the cost of micronutrient sources should be proportional to their effectiveness. However, this is not always the case. Where fertilizer supplier, consultants, or farmers must choose between various sources of micronutrients, one of the major factors in making that choice should be the solubility of each material. Low solubility materials may have some value in a long-term soil build up program, but when immediate results are the goal, highly soluble fertilizers are the best choice. If the fertilizer producer cannot provide information on the solubility of their product, you can send a sample to Spectrum Analytic for that analysis.
Soybean plants (as well as the symbiotic bacteria) require some of each of the essential nutrients. Of the three primary nutrients - N, P, and K - nitrogen is supplied by the symbiotic bacteria in the nodules. The other nutrients come from the soil and will be taken into the plant as it takes up water.
Table 20 shows nutrient uptake requirements for soybeans under maximum economic yield conditions at various stages of growth, based on research by - Dr. Ray Flannery, who raised 101 bu/Ac soybeans in 1986. The research was performed on a Freehold sandy loam soil, testing very high in P and K, with a pH of 6.0 to 6.5. Total nutrient applications were 150 lbs/Ac N, 200 lbs/Ac P2O5, and 300 lbs/Ac K2O applied preplant, and two fertigations. Figure 13 shows nutrient storage in plant parts at the green stage of development.
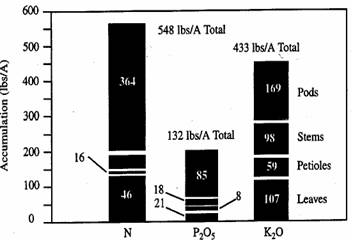
Figure 13. Illustrates the plant parts in which the nutrients are stored at the green stage of development.
| Table 20: Dry Matter Accumulation and Nutrients Absorbed Per Acre by Soybeans at Various Growth Stages | ||||||
|---|---|---|---|---|---|---|
| Growth Stage | (2) Three Tri-leaf | (3) Six Tri-leaf | (5) Full Bloom | (7) Pod Dev. | (9) Soft Green | (10) Mature |
| Days | 40 | 51 | 67 | 82 | 103 | 119 |
| Lbs/Acre | ||||||
| Dry Matter | 784 | 1585 | 4757 | 9495 | 16,900 | 16,594 |
| N | 30 | 46 | 171 | 308 | 548 | 494 |
| P2O5 | 6 | 12 | 40 | 74 | 132 | 112 |
| K2O | 27 | 57 | 149 | 293 | 433 | 397 |
| Ca | 11 | 20 | 49 | 101 | 152 | 165 |
| Mg | 4 | 8 | 20 | 34 | 55 | 56 |
| S | 2 | 3 | 11 | 20 | 32 | 28 |
| Micronutrient uptake (lbs/Acre) | ||||||
| Mn | Fe | Cu | B | Zn | ||
| Near Maturity | 1.03 | 6.32 | 0.12 | 0.08 | 0.74 | |
Source: Dr Roy Flannery
Phosphorus accumulates in the seed and pods while potassium is important to the stems and leaves as well as the pods.
As a consequence, the potassium needs are about twice as great as the phosphorus needs. However, only about half of the phosphorus and potassium needs are for the vegetative growth, whereas the balance is for seed development. Soybeans remove about 0.80 pounds of P2O5 and about 1.40 pounds K2O per bushel of yield harvested.
| Early Soybean Growth and Development | |
|---|---|
| Growth Stage | Time (Days) (Ideal Growing Conditions) |
| First trifoliolate leaf emerges with leaflets on trifoliate above not touching (V1 stage) | 10 to 12 |
| Photosynthesis by the developed leaves is adequate for the plant to sustain itself | 12 to 14 |
| The first (V1) through the fifth (V5) trifoliolate leaves emerge | 5-day intervals |
| V2 Stage • 6 to 8 inches (15.2 to 20.3 cm) • Active nitrogen fixation begins • Lateral roots are proliferating rapidly into the top 6 inches (15.2 cm) of soil V3 Stage • 7 to 9 inches (17.9 to 22.9 cm) V5 Stage • 10 to 12 inches (25.4 to 30.5 cm) |
|
| The sixth (V6) to the last trifoliolate leaf [V(n)] develop (about the beginning of seed development in the pod | 3-day intervals |
| At V6, lateral roots grow completely across a row spacing of 30 inches (76) cm) or less: 50% leaf loss at V6 reduces yield approximately 3% | |