Soil pH Management
Soil pH
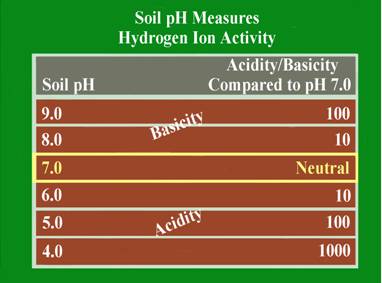 This is a measure of the soil acidity or alkalinity and is sometimes called the soil “water” pH. This is because it is a measure of the pH of the soil solution, which is considered the active pH that affects plant growth. Soil pH is the foundation of essentially all soil chemistry and nutrient reaction and should be the first consideration when evaluating a soil test. The total range of the soil pH scale is from 0-14. Values below the mid-point (pH 7.0) are acidic and those above pH 7.0 are alkaline. A soil pH of 7.0 is considered to be neutral. Most plants perform best in a soil that is slightly acid to neutral (pH 6.0-7.0). Some plants like blueberries require the soil to be more acid (pH 4.5-5.5), and other, like alfalfa will tolerate a slightly alkaline soil (pH 7.0-7.5).
This is a measure of the soil acidity or alkalinity and is sometimes called the soil “water” pH. This is because it is a measure of the pH of the soil solution, which is considered the active pH that affects plant growth. Soil pH is the foundation of essentially all soil chemistry and nutrient reaction and should be the first consideration when evaluating a soil test. The total range of the soil pH scale is from 0-14. Values below the mid-point (pH 7.0) are acidic and those above pH 7.0 are alkaline. A soil pH of 7.0 is considered to be neutral. Most plants perform best in a soil that is slightly acid to neutral (pH 6.0-7.0). Some plants like blueberries require the soil to be more acid (pH 4.5-5.5), and other, like alfalfa will tolerate a slightly alkaline soil (pH 7.0-7.5).
The soil pH scale is logarithmic; meaning that each whole number is a factor of 10 larger or smaller than the ones next to it. For example if a soil has a pH of 7.0 and this pH is lowered to pH 6.0, the acid content of that soil is increased 10-fold. If the pH is lowered further to pH 5.0, the acid content becomes 100 times greater than at pH 7.0. The logarithmic nature of the pH scale means that small changes in a soil pH can have large effects on nutrient availability and plant growth.
Buffer pH (BpH)
 This is a value that is generated in the laboratory; it is not an existing feature of the soil. Laboratories perform this test in order to develop lime recommendations, and it actually has no other practical value.
This is a value that is generated in the laboratory; it is not an existing feature of the soil. Laboratories perform this test in order to develop lime recommendations, and it actually has no other practical value.
In basic terms, the BpH is the resulting sample pH after the laboratory has added a liming material. In this test, the laboratory adds a chemical mixture called a buffering solution. This solution functions like extremely fast-acting lime. Each soil sample receives the same amount of buffering solution; therefore the resulting pH is different for each sample. To determine a lime recommendation, the laboratory looks at the difference between the original soil pH and the ending pH after the buffering solution has reacted with the soil. If the difference between the two pH measurements is large, it means that the soil pH is easily changed, and a low rate of lime will be sufficient. If the soil pH changes only a little after the buffering solution has reacted, it means that the soil pH is difficult to change and a larger lime addition is needed to reach the desired pH for the crop.
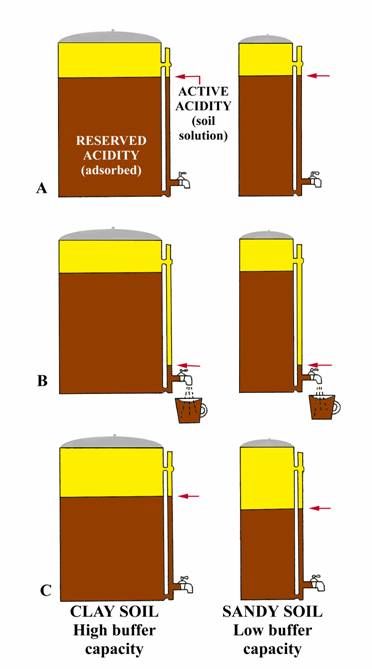
The reasons that a soil may require differing amounts of lime to change the soil pH relates to the soil CEC and the “reserve” acidity that is contained by the soil. Soil acidity is controlled by the amount of hydrogen (H+) and the aluminum (Al+++) ions that are either contained in, or generated by the soil and soil components. Soils with a high CEC have a greater capacity to contain or generate these sources of acidity. Therefore, at a given soil pH, a soil with a higher CEC (thus a lower buffered pH) will normally require more lime to reach a given target pH than a soil with a lower CEC.
The following analogy (adapted from University of Nebraska, Bulletin G74-153) may give a simple explanation. Consider two-coffee pots (figure to right) one 50-cup capacity and one 10 cup, both having the same size indicator tube and spigot. Coffee in the indicator tube represents the active acidity (measured by regular pH) and that coffee in the pot represents the reserve acidity (measured by buffer pH). Let the large pot represent a clay soil high in organic matter while the small pot represents a sandy soil. Both pots have equal amounts of coffee in the indicator tube; i.e., same active hydrogen, so same soil pH. Now open the spigot and remove one-cup of coffee from each pot (figure B). Removing one cup of coffee from each pot could be equated to the addition of small amount of limestone to an acid soil. Opening the spigot will cause the level of coffee in the indicator tube to drop below the level in the pot, but will return to almost the original level (clay soil) when the spigot is closed. The momentary drop of coffee in the indicator tube represents the initial increased in pH when lime is added (affects active hydrogen), but reserve hydrogen (similar to coffee in the pot) soon equalizes the effect from the lime and the pH returns to essentially its original level (clay soil, figure C). Thus, if the pH is 6.5 or lower, a buffer pH is run to measure the reserve acidity. The result of the buffer pH shows the amount of lime required to neutralize a major portion of the reserve acidity. The relative amounts of coffee in the two pots (figure C) show why a sandy soil and clay soil with the same pH result in different lime requirements. For example, the small addition of limestone (equivalent to removing one cup of coffee from each pot) reduced the total coffee (reserve acidity) by 10% in the small pot (sandy soil), but only 2% of the large pot (clay soil). In a similar manner, 1 ton of agricultural limestone will make a greater difference in the pH of a sandy soil than of a clay soil.
Soil pH Management
Lime tables Mineral Soil, High Organic Matter Mineral, and Muck Soil Organic matter 20% or more are selected portions of the lime recommendation calculations. Actual recommendations include many more combinations of Original pH, Target pH, and Buffer pH. Spectrum lime recommendations are expressed in pounds per acre of pure calcium carbonate (CaCO3) per 7 inch depth and typical fineness of grind. Make appropriate adjustments for the local lime source (see adjustments following lime tables).
| Mineral Soil: Typical soils with organic matter between 0% and 10% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Lime (CaCO3) Recommendations | |||||||||||||
| Original pH | Target pH | Lb./Acre | Original pH | Target pH | Lb./1000 ft.2 | ||||||||
| Soil Buffer pH (BpH) | Soil Buffer pH (BpH) | ||||||||||||
| 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | ||||
| 4.5 | 5.0 | 11982 | 9427 | 6511 | 3776 | 1040 | 4.5 | 5.0 | 275 | 216 | 149 | 87 | 24 |
| 4.5 | 5.5 | 16827 | 12981 | 9136 | 5290 | 1444 | 4.5 | 5.5 | 386 | 298 | 210 | 121 | 33 |
| 4.5 | 6.0 | 20227 | 15602 | 10987 | 6353 | 1728 | 4.5 | 6.0 | 464 | 358 | 252 | 146 | 40 |
| 4.5 | 6.5 | 22891 | 17656 | 12420 | 7185 | 1950 | 4.5 | 6.5 | 526 | 405 | 285 | 165 | 45 |
| 5.0 | 6.0 | 16795 | 12949 | 9104 | 5258 | 1412 | 5.0 | 6.0 | 386 | 297 | 209 | 121 | 32 |
| 5.0 | 6.5 | 20195 | 15570 | 10946 | 6321 | 1696 | 5.0 | 6.5 | 464 | 357 | 251 | 145 | 39 |
| 5.5 | 6.0 | 0 | 9205 | 6470 | 3735 | 999 | 5.5 | 6.0 | 0 | 211 | 149 | 86 | 23 |
| 5.5 | 6.5 | 0 | 12940 | 9095 | 5249 | 1403 | 5.5 | 6.5 | 0 | 297 | 209 | 121 | 32 |
| 6.0 | 6.5 | 0 | 0 | 6468 | 3732 | 997 | 6.0 | 6.5 | 0 | 0 | 148 | 86 | 23 |
| High Organic Matter Mineral - Soil O.M. Between 10% and 19.9% | ||||
|---|---|---|---|---|
| Sample Lime (CaCO3) Recommendations | ||||
| Original. pH | Target. pH | (Lb./Acre) | ||
| Soil Buffer pH (BpH) | ||||
| 4.5 | 5.0 | 5.5 | ||
| 4.5 | 6.0 | 24852 | 20227 | 15602 |
| 5.0 | 6.0 | 0 | 16795 | 12949 |
| 5.5 | 6.0 | 0 | 0 | 9205 |
| Muck Soil Organic Matter 20% or more | |||
|---|---|---|---|
| Sample Lime (CaCO3) Recommendations | |||
| Original pH | Target pH | Lb./Acre | |
| Soil Buffer pH (BpH) | |||
| 4.5 | 5.0 | ||
| 4.5 | 5.2 | 17422 | 14183 |
| 5.0 | 5.2 | 0 | 7302 |
Steps to Convert CaCO3 Recommendations to Local Ag Lime
Spectrum lime recommendations are expressed in terms of 100% pure Calcium carbonate (CaCO3) equivalent (CCE). They assume a 6.75 inch tillage depth and a lime fineness of 50% - 70% through a 60 mesh screed. If local lime does not meet these criteria, use the following steps to adjust final recommendations.
| Step 1: Tillage Depth Adjustment Factors: (Multiply CaCO3 rate by the factor listed across from appropriate plow depth) |
|
|---|---|
| Effective Tillage Depth (inches) | Multiplying Factor |
| 0 - 3 | 0.40 |
| 6 | 0.86 |
| 7 | 1.00 |
| 8 | 1.14 |
| 9 | 1.29 |
| 10 | 1.43 |
| 11 | 1.57 |
| 12 | 1.71 |
| Step 2: Lime Type/Purity Adjustment Factors: (Select most appropriate lime type or purity and multiply the results of step 1 by the factor listed across from appropriate type or purity). |
|
|---|---|
| Lime Type, Purity or Analysis | Factor |
| 90% to 110% (CCE or TNP) | 1.00 |
| 80% to 89% | 1.17 |
| 70% to 79% | 1.33 |
| 60% to 69% | 1.54 |
| 50% to 59% | 1.81 |
| 100% pure CaCO3 (40% Ca) | 1.00 |
| Dolomitic Lime | Factor |
| 50% CaCO3 + 50% MgCO3 (22%Ca + 15%Mg) | 0.92 |
| 75% CaCO3 + 25% MgCO3 (31%Ca + 7% Mg) | 0.96 |
| Other Materials | Factor |
| *Calcium oxide (burnt lime) | 0.56 |
| *Calcium hydroxide (hydrated lime) | 0.74 |
| Granulated slag | 1.00 |
| *These materials may achieve the target soil pH in 1 to 12 days after application. | |
| Step 3: Adjustments For Lime Grind Fineness: (Select the multiplying factor across from the applicable screen size, and multiply the results of step 2 by that factor). |
||
|---|---|---|
| % Passing Through Screen Size | Multiplying Factor | |
| 100 Mesh | 60 Mesh | |
| 80-100 | 95-100 | 0.80 |
| 60-79 | 70-94 | 0.85 |
| 40-59 | 50-69 | 1.00 |
| 30-39 | 50-69 | 1.25 |
| 20-29 | 30-39 | 1.45 |
| 10-19 | 20-29 | 1.70 |
| 0-9 | 0-19 | 2.00 |
Acidifying Soils
Acidifying a field soil is normally an expensive process and only economical for the highest value crops, or very small areas. Because of this, the recommendation program does not automatically make recommendations for acidifying soils.
The following tables, adapted from various sources, list typical rates of elemental S required to reduce soil pH, plus conversion factors for common acidifying materials. The values listed are approximate, and individual soils may behave somewhat differently. Re-test the soil annually to monitor the amount of pH change caused by applications. Information from North Carolina indicates that low CEC soils that have an acid sub-soil could experience an excessive initial pH drop from rates of S higher than 300 lb./acre (0.69 lb/100 sq. ft.). Their guidelines indicate that crop damage has resulted from this effect. On such soils, it would be wise to make multiple, small applications of S, and monitor the pH change to avoid problems.
| Sulfur Effect on Soil pH (lb.-S/acre) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Original pH | Target pH | Soil CEC | ||||||
| 1 | 5 | 10 | 15 | 20 | 25 | 35 | ||
| 5.0 | 4.5 | 88 | 175 | 353 | 530 | 665 | 800 | 1120 |
| 5.5 | 4.5 | 175 | 350 | 700 | 1050 | 1325 | 1600 | 2234 |
| 6.0 | 4.5 | 265 | 530 | 1035 | 1540 | 1925 | 2310 | 3228 |
| 6.5 | 4.5 | 330 | 660 | 1340 | 2020 | 2525 | 3030 | 4251 |
| 7.0 | 4.5 | 420 | 840 | 1695 | 2550 | 3190 | 3830 | 5368 |
| 7.5 | 4.5 | 501 | 1002 | 2004 | 3006 | 3758 | 4509 | 6317 |
| 6.0 | 5.0 | 110 | 220 | 335 | 450 | 550 | 650 | 885 |
| 6.5 | 5.0 | 274 | 548 | 943 | 1337 | 1644 | 1951 | 2701 |
| 7.0 | 5.0 | 374 | 747 | 1257 | 1766 | 2168 | 2569 | 3547 |
| 7.5 | 5.0 | 469 | 937 | 1547 | 2157 | 2642 | 3127 | 4309 |
| 8.0 | 5.0 | 845 | 1689 | 2024 | 2357 | 2641 | 2924 | 3849 |
| 6.0 | 5.5 | 109 | 218 | 272 | 327 | 382 | 436 | 576 |
| 6.5 | 5.5 | 218 | 436 | 545 | 654 | 763 | 872 | 1151 |
| 7.0 | 5.5 | 327 | 654 | 818 | 981 | 1145 | 1308 | 1726 |
| 7.5 | 5.5 | 436 | 872 | 1090 | 1308 | 1526 | 1744 | 2301 |
| 8.0 | 5.5 | 763 | 1526 | 1799 | 2071 | 2344 | 2616 | 3414 |
| 8.5 | 5.5 | 1090 | 2180 | 2507 | 2834 | 3161 | 3488 | 4526 |
| 7.0 | 6.0 | 189 | 377 | 472 | 566 | 685 | 804 | 1051 |
| 7.5 | 6.0 | 343 | 686 | 870 | 1054 | 1213 | 1372 | 1825 |
| 8.0 | 6.0 | 682 | 1363 | 1575 | 1786 | 2047 | 2308 | 2980 |
| 8.5 | 6.0 | 1045 | 2090 | 2379 | 2667 | 2956 | 3244 | 4199 |
| 7.0 | 6.5 | 50 | 100 | 125 | 150 | 225 | 300 | 375 |
| 7.5 | 6.5 | 250 | 500 | 650 | 800 | 900 | 1000 | 1349 |
| 8.0 | 6.5 | 600 | 1200 | 1350 | 1500 | 1750 | 2000 | 2545 |
| 8.5 | 6.5 | 1000 | 2000 | 2250 | 2500 | 2750 | 3000 | 3871 |
| 8.0 | 7.0 | 519 | 1037 | 1126 | 1215 | 1453 | 1692 | 2111 |
| 8.0 | 7.5 | 437 | 874 | 901 | 929 | 1156 | 1384 | 1676 |
Adapted from the Western Fertilizer Handbook 7th ed.: Nursery Management, 2nd ed. H. Davidson, et al., 1988; the Highbush Blueberry Production Guide (NRAES-55), Northeast Regional Agricultural Engineering Service, M. Pritts and J. Hancock ed., 1992.; and Vegetable Growing Handbook, W. E. Splittstoesser, 1979.
| Sulfur Effect on Soil pH (lb.-S/100 ft.2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Original pH | Target pH | Soil CEC | ||||||
| 1 | 5 | 10 | 15 | 20 | 25 | 35 | ||
| 5.0 | 4.5 | 0.20 | 0.40 | 0.81 | 1.22 | 1.53 | 1.84 | 2.57 |
| 5.5 | 4.5 | 0.40 | 0.80 | 1.61 | 2.41 | 3.04 | 3.67 | 5.13 |
| 6.0 | 4.5 | 0.61 | 1.22 | 2.38 | 3.54 | 4.42 | 5.30 | 7.41 |
| 6.5 | 4.5 | 0.76 | 1.52 | 3.08 | 4.64 | 5.80 | 6.96 | 9.76 |
| 7.0 | 4.5 | 0.96 | 1.93 | 3.89 | 5.85 | 7.32 | 8.79 | 12.32 |
| 7.5 | 4.5 | 1.15 | 2.30 | 4.60 | 6.90 | 8.63 | 10.35 | 14.50 |
| 6.0 | 5.0 | 0.25 | 0.51 | 0.77 | 1.03 | 1.26 | 1.49 | 2.03 |
| 6.5 | 5.0 | 0.63 | 1.26 | 2.16 | 3.07 | 3.77 | 4.48 | 6.20 |
| 7.0 | 5.0 | 0.86 | 1.71 | 2.89 | 4.05 | 4.98 | 5.90 | 8.14 |
| 7.5 | 5.0 | 1.08 | 2.15 | 3.55 | 4.95 | 6.07 | 7.18 | 9.89 |
| 8.0 | 5.0 | 1.94 | 3.88 | 4.65 | 5.41 | 6.06 | 6.71 | 8.84 |
| 6.0 | 5.5 | 0.25 | 0.50 | 0.62 | 0.75 | 0.88 | 1.00 | 1.32 |
| 6.5 | 5.5 | 0.50 | 1.00 | 1.25 | 1.50 | 1.75 | 2.00 | 2.64 |
| 7.0 | 5.5 | 0.75 | 1.50 | 1.88 | 2.25 | 2.63 | 3.00 | 3.96 |
| 7.5 | 5.5 | 1.00 | 2.00 | 2.50 | 3.00 | 3.50 | 4.00 | 5.28 |
| 8.0 | 5.5 | 1.75 | 3.50 | 4.13 | 4.75 | 5.38 | 6.01 | 7.84 |
| 8.5 | 5.5 | 2.50 | 5.00 | 5.76 | 6.51 | 7.26 | 8.01 | 10.39 |
| 7.0 | 6.0 | 0.43 | 0.87 | 1.08 | 1.30 | 1.57 | 1.85 | 2.41 |
| 7.5 | 6.0 | 0.79 | 1.57 | 2.00 | 2.42 | 2.78 | 3.15 | 4.19 |
| 8.0 | 6.0 | 1.57 | 3.13 | 3.62 | 4.10 | 4.70 | 5.30 | 6.84 |
| 8.5 | 6.0 | 2.40 | 4.80 | 5.46 | 6.12 | 6.79 | 7.45 | 9.64 |
| 7.0 | 6.5 | 0.11 | 0.23 | 0.29 | 0.34 | 0.52 | 0.69 | 0.86 |
| 7.5 | 6.5 | 0.57 | 1.15 | 1.49 | 1.84 | 2.07 | 2.30 | 3.10 |
| 8.0 | 6.5 | 1.38 | 2.75 | 3.10 | 3.44 | 4.02 | 4.59 | 5.84 |
| 8.5 | 6.5 | 2.30 | 4.59 | 5.17 | 5.74 | 6.31 | 6.89 | 8.89 |
| 8.0 | 7.0 | 1.19 | 2.38 | 2.58 | 2.79 | 3.34 | 3.88 | 4.85 |
| 8.0 | 7.5 | 1.00 | 2.01 | 2.07 | 2.13 | 2.65 | 3.18 | 3.85 |
Adapted from the Western Fertilizer Handbook 7th ed.: Nursery Management, 2nd ed. H. Davidson, et al., 1988; the Highbush Blueberry Production Guide (NRAES-55), Northeast Regional Agricultural Engineering Service, M. Pritts and J. Hancock ed., 1992.; and Vegetable Growing Handbook, W. E. Splittstoesser, 1979.
| Common Acidifying Materials | |||
|---|---|---|---|
| Material | Chemical Formula | Percent Sulfur | Lbs of Material to Equal 100 Lbs of Sulfur |
| Sulfur* | S | 99.0 | 100 |
| Sulfuric Acid | H2SO4 | 32.0 | 306 |
| Sulfur Dioxide | SO2 | 50.0 | 198 |
| Iron Sulfate | FeSO4.7H2O | 11.5 | 896 |
| Aluminum Sulfate | Al2(SO4)3 | 14.4 | 694 |
| Ammonium Sulfate | (NH4)2SO4 | 23.7 | 422 |
| *NOTE: The acidifying effect of elemental sulfur is caused by sulfur oxidizing bacteria. These bacteria must be present in the soil, in sufficient amounts, in order to have the desired effect. If a soils pH is above 7.2 in its natural state. it may not have a large population of sulfur oxidizing bacteria. In these cases it may be helpful to inoculate it by adding some soil from another source that is naturally acid. Also, the pH change caused by the bacterial oxidation of sulfur may be relatively slow (12 months or more) since they are dependent on sufficient soil moisture and temperature to accomplish efficient sulfur oxidation. The other products listed produce a chemical acidifying effect, independent of soil organisms and may be faster and more dependable than elemental sulfur. | |||
| Calculated Equivalent Acidity of Common Nitrogen Materials | ||||
|---|---|---|---|---|
| N Source | % N | Chemical Formula | 100 Lb of Nitrogen* | 100 Lb of Fertilizer* |
| Ammonium Sulfate | 21 | (NH4)2SO4 | 535 | 151 |
| Anhydrous Ammonia | 82 | NH3 | 180 | 295 |
| Ammonium Nitrate | 34 | NH4NO3 | 180 | 122 |
| Urea | 46 | CO(NH2)2 | 180 | 166 |
| UAN | 28-32 | CO(NH2)2+NH4NO3 | 180 | 101-115 |
| Calcium Nitrate | 15 | Ca(NO3)2 | 135B | 20B |
| Sodium Nitrate | 16 | NaNO3 | 180B | 29B |
| Potassium Nitrate | 13 | KNO3 | 200B | 26B |
| Adapted from the Potash and Phosphate Institutes Soil Fertility Manual | ||||
| * Pounds of calcium carbonate (CaCO3) needed to neutralize the acidity formed from 100 pounds of nitrogen, or nitrogen containing fertilizer. The "B" denotes a basic (pH increasing) effect. These are theoretical values and may differ somewhat in actual soil. | ||||